Abstract
Introduction Each year, the Center for International Blood and Marrow Transplant Research (CIBMTR) reports the outcomes of hematopoietic cell transplants (HCTs) at transplant centers (TCs) across the United States through its Center Specific Analysis (CSA). The CSA estimates the predicted 1-year overall survival (OS) rate and confidence limits for each TC based on its recipient case mix from the preceding 3 years on a rolling basis, then compares the estimated confidence limit to the actual OS rate. Centers’ performance is rated as 0 (OS as expected), −1 (OS worse than expected), or +1 (OS better than expected); and is made publicly available to HCT recipients, providers, and the public. Public reporting of CSA scores for individual TCs could have implications at the patient, TC, and payer levels. It may affect the patient selection process, the volume of HCTs performed, and the outcomes. We hypothesized that a decrease in CSA score would be associated with a subsequent decrease in HCT volume at the index TC while increasing HCT numbers at neighboring TCs.
Methods All TCs that serve adult or combined adult and pediatric populations, that had reported CSA scores, and that had a 3-year average volume of ≥10 allogeneic HCTs from 2012 to 2018 were included in the analysis. The primary outcome was the HCT volume, defined as the number of allogeneic HCTs performed each year at the TC for any indication. A linear mixed model with an unstructured correlation matrix was fitted to the TC volume data to account for repeated longitudinal measurements. Log transformation of the TC volume was used to reduce skewness. The resulting coefficients of the mixed model were transformed using the exponential function to enable them to be interpreted as the ratio of means between groups. Covariates included the prior-year TC volume, the prior-year CSA score (−1, 0, or 1), whether the CSA score changed from the prior year, the calendar year, the TC type (adult only vs. combined adult and pediatric patients), and the years of experience with HCT. Also evaluated was the impact of CSA scores on HCT volume at neighboring TCs, defined as TCs within 45 miles of the index TC or the next closest center if there was no other TC within 45 miles.
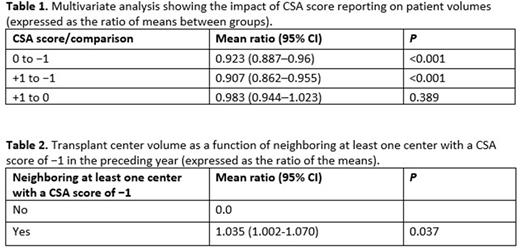
Results We included 91 centers in the analysis, of which 68 had at least one CSA score change during the study period. A prior-year CSA score of −1, as compared with a CSA score of 0, was associated with an 8% reduction in the mean TC volume (mean ratio: 0.92; 95% confidence interval [CI]: 0.89-0.96; P < 0.001) (Table 1) while adjusting for yearly trends and increases in transplant numbers across TCs. Likewise, a prior-year CSA score of −1, as compared with a CSA score of +1, was associated with an 9% reduction in the mean TC volume (mean ratio: 0.91; 95% CI: 0.86-0.96; P < 0.001) (Table 1). There was no significant difference between the mean volumes for prior-year CSA scores of 0 and +1. There was no interaction between the effect of the prior-year CSA score and the HCT volume at a center (≥40 vs. <40 allogeneic HCTs per year, P = 0.3). Being a TC neighboring at least one index TC with a −1 CSA score, as compared with not neighboring at least one index TC with a −1 CSA score, was associated with a 3.5% increase in mean TC volume (mean ratio: 1.035; 95% CI: 1.002-1.07; P = 0.04) (Table 2).
Conclusion Public reporting of CSA scores is associated with changes in the number of HCTs at TCs. A CSA score of −1 is associated with a decrease in volume at the index center and with an associated increase in volume at neighboring centers. Additional research into the causes of this shift in patient volume and the impact on outcomes is needed.
Disclosures
Sharma:Vindico Medical Education: Honoraria; Vertex Pharmaceuticals/CRISPR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Other; CRISPR Therapeutics: Research Funding; Spotlight Therapeutics: Consultancy; Magenta Therapeutics: Other: Research collaboration; Novartis: Other: Other; Medexus Inc: Consultancy. Troy:Navitas Clinical Research: Honoraria; Synthetic Biologics: Honoraria; CryoCell International.: Patents & Royalties; Gamida-Cell Ltd: Consultancy, Honoraria; SinoCell Techologies: Patents & Royalties; The EMMES Corporation: Honoraria; Aegis-CN LLC: Consultancy; The Community Data Roundtable: Consultancy. Bhatt:Pfizer Inc.: Divested equity in a private or publicly-traded company in the past 24 months; Moderna Inc.: Divested equity in a private or publicly-traded company in the past 24 months; Johnson & Johnson: Divested equity in a private or publicly-traded company in the past 24 months; Rite Aid Corp.: Divested equity in a private or publicly-traded company in the past 24 months. Wood:Pfizer: Research Funding; Genentech: Research Funding; Teladoc: Consultancy. Rizzo:Optum Stem Cell: Other: Compensation as participant on expert panel to provide input /information about the center specific survival analysis based on expertise. Compensation for this effort (~$1000) is directed to MCW rather than personally..
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal